Design Therapeutics (DSGN) Price Target Increased by 18.30% to 27.03

The average one-year price target for Design Therapeutics (NASDAQ:DSGN) has been revised to 27.03 / share. This is an increase of 18.30% from the prior estimate of 22.85 dated June 1, 2023.
The price target is an average of many targets provided by analysts. The latest targets range from a low of 19.19 to a high of 44.10 / share. The average price target represents an increase of 312.67% from the latest reported closing price of 6.55 / share.
What is the Fund Sentiment?
There are 231 funds or institutions reporting positions in Design Therapeutics.
This is an increase
of
2
owner(s) or 0.87% in the last quarter.
Average portfolio weight of all funds dedicated to DSGN is 0.10%,
a decrease
of 44.50%.
Total shares owned by institutions decreased
in the last three months by 4.49% to 40,349K shares.
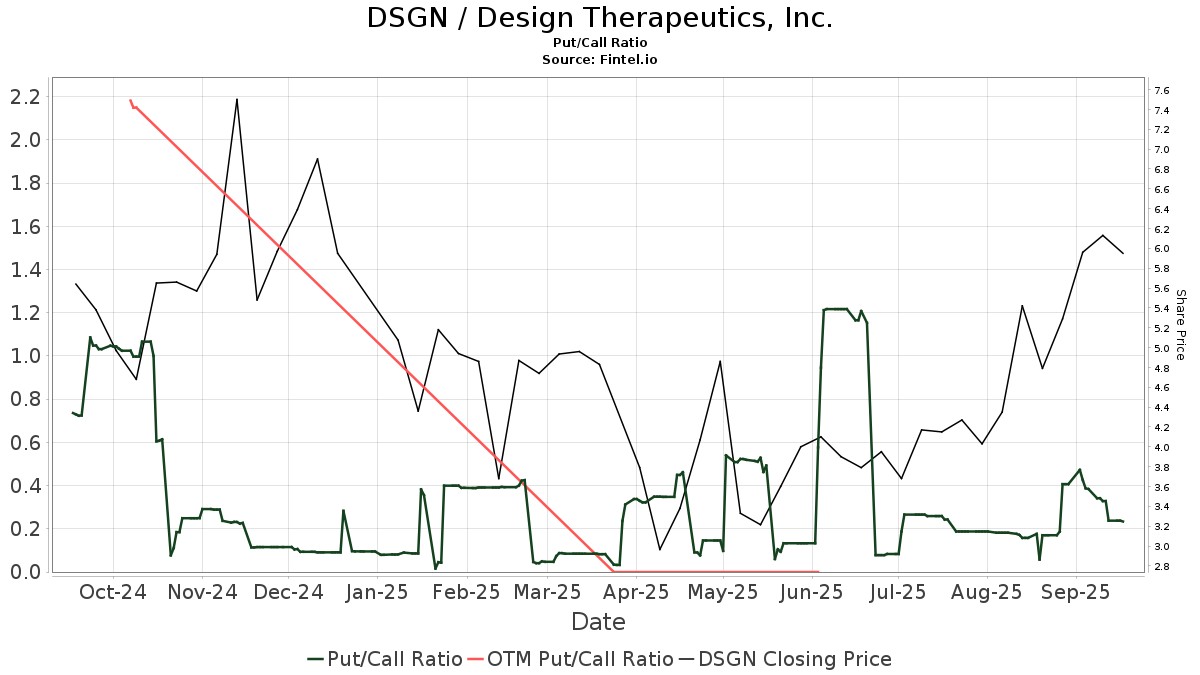 The put/call ratio of DSGN is 0.35, indicating a
bullish
outlook.
The put/call ratio of DSGN is 0.35, indicating a
bullish
outlook.
What are Other Shareholders Doing?

Sr One Capital Management holds 6,526K shares representing 11.67% ownership of the company. No change in the last quarter.
Cormorant Asset Management holds 5,150K shares representing 9.21% ownership of the company. No change in the last quarter.
Avoro Capital Advisors holds 3,500K shares representing 6.26% ownership of the company. In it's prior filing, the firm reported owning 2,272K shares, representing an increase of 35.08%. The firm decreased its portfolio allocation in DSGN by 14.06% over the last quarter.
Ra Capital Management holds 2,971K shares representing 5.31% ownership of the company. No change in the last quarter.
Citadel Advisors holds 2,865K shares representing 5.12% ownership of the company. In it's prior filing, the firm reported owning 2,503K shares, representing an increase of 12.66%. The firm decreased its portfolio allocation in DSGN by 39.38% over the last quarter.
Design Therapeutics Background Information
(This description is provided by the company.)
Design Therapeutics is a biotechnology company developing a new class of therapies based on a platform of gene targeted chimera (GeneTAC™) small molecules. The company’s lead program is focused on the treatment of Friedreich ataxia, followed by a program in myotonic dystrophy type-1 and discovery efforts for multiple other serious degenerative disorders caused by nucleotide repeat expansions.
Additional reading:
- Design Therapeutics Provides Pipeline Updates and Reports First Quarter 2023 Financial Results Initial Data from Phase 1 Multiple-Ascending Dose Trial of DT-216 for Friedreich Ataxia Expected in the Third Quarter of 2023 Progress Across GeneTAC™ Smal
- JOINT FILING AGREEMENT
- Design Therapeutics Highlights Pipeline Progress and Reports Fourth Quarter and Full Year 2022 Financial Results Phase 1 Multiple-Ascending Dose Trial of DT-216 for Friedreich Ataxia Ongoing with Data Expected Mid-Year Continued Progress Across Pipel
- JOINT FILING AGREEMENT
- Trade Date






























